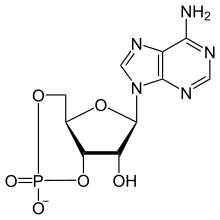
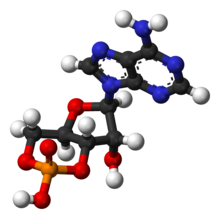
Non Cyclic Amp Lewis Structure
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name (4aR,6R,viiR,7aDue south)-6-(6-Amino-9H-purin-nine-yl)-ii,7-dihydroxytetrahydro-iiH,4H-2λ5-furo[3,2-d][1,three,two]dioxaphosphinin-2-one | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.000.448 |
| IUPHAR/BPS |
|
| KEGG |
|
| MeSH | Cyclic+AMP |
| PubChem CID |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| InChI
| |
| SMILES
| |
| Backdrop | |
| Chemical formula | C10H11NvOviP |
| Tooth mass | 329.206 yard/mol |
| Except where otherwise noted, information are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Cyclic adenosine monophosphate (army camp, circadian AMP, or iii',five'-cyclic adenosine monophosphate) is a second messenger of import in many biological processes. army camp is a derivative of adenosine triphosphate (ATP) and used for intracellular bespeak transduction in many different organisms, conveying the cAMP-dependent pathway.
History [edit]
Earl Sutherland of Vanderbilt Academy won a Nobel Prize in Physiology or Medicine in 1971 "for his discoveries concerning the mechanisms of the action of hormones", specially epinephrine, via second messengers (such equally cyclic adenosine monophosphate, cyclic AMP).
Synthesis [edit]
Cyclic AMP is synthesized from ATP by adenylate cyclase located on the inner side of the plasma membrane and anchored at various locations in the interior of the cell.[1] Adenylate cyclase is activated past a range of signaling molecules through the activation of adenylate cyclase stimulatory Yard (Gsouthward)-protein-coupled receptors. Adenylate cyclase is inhibited by agonists of adenylate cyclase inhibitory Yard (Thoui)-protein-coupled receptors. Liver adenylate cyclase responds more strongly to glucagon, and muscle adenylate cyclase responds more strongly to adrenaline.
army camp decomposition into AMP is catalyzed past the enzyme phosphodiesterase.
Functions [edit]
campsite is a second messenger, used for intracellular signal transduction, such as transferring into cells the effects of hormones like glucagon and adrenaline, which cannot pass through the plasma membrane. Information technology is also involved in the activation of poly peptide kinases. In add-on, army camp binds to and regulates the function of ion channels such as the HCN channels and a few other circadian nucleotide-binding proteins such equally Epac1 and RAPGEF2.
Role in eukaryotic cells [edit]
cAMP is associated with kinases function in several biochemical processes, including the regulation of glycogen, sugar, and lipid metabolism.[2]
In eukaryotes, cyclic AMP works by activating poly peptide kinase A (PKA, or cAMP-dependent poly peptide kinase). PKA is normally inactive as a tetrameric holoenzyme, consisting of two catalytic and ii regulatory units (C2Rtwo), with the regulatory units blocking the catalytic centers of the catalytic units.
Cyclic AMP binds to specific locations on the regulatory units of the protein kinase, and causes dissociation between the regulatory and catalytic subunits, thus enabling those catalytic units to phosphorylate substrate proteins.
The active subunits catalyze the transfer of phosphate from ATP to specific serine or threonine residues of protein substrates. The phosphorylated proteins may act directly on the cell's ion channels, or may become activated or inhibited enzymes. Poly peptide kinase A can also phosphorylate specific proteins that bind to promoter regions of DNA, causing increases in transcription. Not all protein kinases respond to military camp. Several classes of protein kinases, including protein kinase C, are not cAMP-dependent.
Further effects mainly depend on camp-dependent poly peptide kinase, which vary based on the type of cell.
Still, there are some pocket-sized PKA-independent functions of army camp, due east.chiliad., activation of calcium channels, providing a modest pathway by which growth hormone-releasing hormone causes a release of growth hormone.
However, the view that the majority of the furnishings of campsite are controlled by PKA is an outdated one. In 1998 a family of army camp-sensitive proteins with guanine nucleotide exchange cistron (Gef) activity was discovered. These are termed Exchange proteins activated by military camp (Epac) and the family unit comprises Epac1 and Epac2.[iii] The mechanism of activation is like to that of PKA: the Gef domain is usually masked by the N-concluding region containing the cAMP binding domain. When cAMP binds, the domain dissociates and exposes the now-active Global environment facility domain, assuasive Epac to activate small Ras-similar GTPase proteins, such as Rap1.
[edit]
In the species Dictyostelium discoideum, campsite acts outside the cell every bit a secreted point. The chemotactic aggregation of cells is organized by periodic waves of cAMP that propagate between cells over distances as big equally several centimetres. The waves are the event of a regulated production and secretion of extracellular army camp and a spontaneous biological oscillator that initiates the waves at centers of territories.[four]
Office in leaner [edit]
In bacteria, the level of cAMP varies depending on the medium used for growth. In particular, military camp is low when glucose is the carbon source. This occurs through inhibition of the military camp-producing enzyme, adenylate cyclase, as a side-effect of glucose transport into the cell. The transcription factor cAMP receptor protein (CRP) also chosen CAP (catabolite gene activator poly peptide) forms a complex with campsite and thereby is activated to demark to DNA. CRP-camp increases expression of a large number of genes, including some encoding enzymes that can supply energy contained of glucose.
campsite, for example, is involved in the positive regulation of the lac operon. In an surround with a low glucose concentration, campsite accumulates and binds to the allosteric site on CRP (cAMP receptor protein), a transcription activator protein. The protein assumes its active shape and binds to a specific site upstream of the lac promoter, making it easier for RNA polymerase to bind to the adjacent promoter to outset transcription of the lac operon, increasing the rate of lac operon transcription. With a loftier glucose concentration, the cAMP concentration decreases, and the CRP disengages from the lac operon.
Pathology [edit]
Since circadian AMP is a 2nd messenger and plays vital role in jail cell signalling, it has been implicated in various disorders but non restricted to the roles given below:
Role in human carcinoma [edit]
Some research has suggested that a deregulation of camp pathways and an abnormal activation of army camp-controlled genes is linked to the growth of some cancers.[five] [6] [seven]
Function in prefrontal cortex disorders [edit]
Recent research suggests that military camp affects the office of higher-order thinking in the prefrontal cortex through its regulation of ion channels called hyperpolarization-activated cyclic nucleotide-gated channels (HCN). When cAMP stimulates the HCN, the channels open, endmost the encephalon cell to communication and thus interfering with the function of the prefrontal cortex. This enquiry, peculiarly the cognitive deficits in age-related illnesses and ADHD, is of interest to researchers studying the brain.[eight]
military camp is involved in activation of trigeminocervical system leading to neurogenic inflammation and causing migraine.[ commendation needed ]
Role in infectious disease agents' pathogenesis [edit]
Disrupted functioning of campsite has been noted as ane of the mechanisms of several bacterial exotoxins.
They can be subgrouped into 2 distinct categories:[9]
- Toxins that interfere with enzymes ADP-ribosyl-transferases, and
- invasive adenylate cyclases.
[edit]
- Cholera toxin is an AB toxin that has five B subints and one A subunit. The toxin acts by the following mechanism: Beginning, the B subunit ring of the cholera toxin binds to GM1 gangliosides on the surface of target cells. If a cell lacks GM1 the toxin most likely binds to other types of glycans, such as Lewis Y and Lewis X, attached to proteins instead of lipids.[x] [eleven] [12] [9]
Uses [edit]
Forskolin is ordinarily used as a tool in biochemistry to raise levels of cAMP in the study and research of cell physiology.[thirteen]
See also [edit]
- Cyclic guanosine monophosphate (cGMP)
- viii-Bromoadenosine 3',5'-cyclic monophosphate (eight-Br-military camp)
- Acrasin specific to chemotactic apply in Dictyostelium discoideum.
- phosphodiesterase 4 (PDE 4) which degrades cAMP
References [edit]
- ^ Rahman N, Cadet J, Levin LR (November 2013). "pH sensing via bicarbonate-regulated "soluble" adenylate cyclase (sAC)". Front Physiol. 4: 343. doi:x.3389/fphys.2013.00343. PMC3838963. PMID 24324443.
- ^ Ali ES, Hua J, Wilson CH, Tallis GA, Zhou FH, Rychkov GY, Barritt GJ (2016). "The glucagon-like peptide-1 analogue exendin-4 reverses impaired intracellular Ca2+ signalling in steatotic hepatocytes". Biochimica et Biophysica Acta (BBA) - Molecular Jail cell Enquiry. 1863 (9): 2135–46. doi:10.1016/j.bbamcr.2016.05.006. PMID 27178543.
- ^ Bos, Johannes Fifty. (Dec 2006). "Epac proteins: multi-purpose military camp targets". Trends in Biochemical Sciences. 31 (12): 680–686. doi:x.1016/j.tibs.2006.x.002. PMID 17084085.
- ^ Anderson, Peter A. V. (2013-eleven-eleven). Evolution of the First Nervous Systems. Springer Science & Business Media. ISBN978-one-4899-0921-3.
- ^ Abramovitch, Rinat; Tavor, Einat; Jacob-Hirsch, Jasmine; Zeira, Evelyne; Amariglio, Ninette; Pappo, Orit; Rechavi, Gideon; Galun, Eithan; Honigman, Alik (xv February 2004). "American Clan for Cancer Enquiry (military camp-responsive Genes and Tumor Progression)". Cancer Research. 64 (4): 1338–1346. doi:x.1158/0008-5472.CAN-03-2089. PMID 14973073. S2CID 14047485.
- ^ Dumaz, Nicolas; Hayward, Robert; Martin, Jan; Ogilvie, Lesley; Hedley, Douglas; Curtin, John A.; Bastian, Boris C.; Springer, Caroline; Marais, Richard (October 2006). "American Association for Cancer Research (military camp Dysregulation and Melonoma)". Cancer Inquiry. 66 (nineteen): 9483–9491. doi:10.1158/0008-5472.CAN-05-4227. PMID 17018604.
- ^ Simpson, B. J.; Ramage, A. D.; Hulme, M. J.; Burns, D. J.; Katsaros, D.; Langdon, Due south. P.; Miller, W. R. (January 1996). "American Association for Cancer Research (army camp-binding Proteins' Presence in Tumors)". Clinical Cancer Research. 2 (i): 201–206.
- ^ "ScienceDaily ::Brain Networks Strengthened By Closing Ion Channels, Inquiry Could Pb To ADHD Treatment".
- ^ a b Kather, H; Aktories, K (November xv, 1983). "cAMP-System und bakterielle Toxine [The army camp organisation and bacterial toxins]". Klin Wochenschr. 61 (22): 1109–1114. doi:10.1007/BF01530837. PMID 6317939. S2CID 33162709. Retrieved Feb 26, 2022.
- ^ Amberlyn 1000 Wands; Akiko Fujita (Oct 2015). "Fucosylation and protein glycosylation create functional receptors for cholera toxin". eLife. Vol. iv. doi:10.7554/eLife.09545.
- ^ Cervin J, Wands AM, Casselbrant A, Wu H, Krishnamurthy S, Cvjetkovic A, et al. (2018) GM1 ganglioside-contained intoxication by Cholera toxin. PLoS Pathog xiv(two): e1006862. https://doi.org/10.1371/journal.ppat.1006862
- ^ Fucosylated Molecules Competitively Interfere with Cholera Toxin Bounden to Host Cells; Amberlyn Thousand. Wands, Jakob Cervin, He Huang, Ye Zhang, Gyusaang Youn, Chad A. Brautigam, Maria Matson Dzebo, Per Björklund, Ville Wallenius, Danielle K. Bright, Clay Due south. Bennett, Pernilla Wittung-Stafshede, Nicole S. Sampson, Ulf Yrlid, and Jennifer J. Kohler; ACS Infectious Diseases Commodity ASAP, DOI: 10.1021/acsinfecdis.7b00085
- ^ Alasbahi, RH; Melzig, MF (January 2012). "Forskolin and derivatives as tools for studying the function of cAMP". Die Pharmazie. 67 (one): five–thirteen. PMID 22393824.
Additional images [edit]
-
cAMP represented in iii ways
-
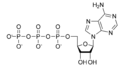
Non Cyclic Amp Lewis Structure,
Source: https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate
Posted by: williamsplevelit.blogspot.com


0 Response to "Non Cyclic Amp Lewis Structure"
Post a Comment